

3) If possible reduce subscripts to give a ratio with smallest whole number. Diatomic ions are molecules made up of two atoms that have a net charge greater or less than zero, i.e., these kinds of molecules either have additional electrons or are missing one or more. High-purity iodine is not needed in our propulsion system, and the total propellant cost for a purity of 99.5 was approximately US60, with an additional cost below US. 2)Switch the charge for each ion to the subscript of the other ion. ChemTalk chemistry tutorial- including cations, anions and polyatomic ions.
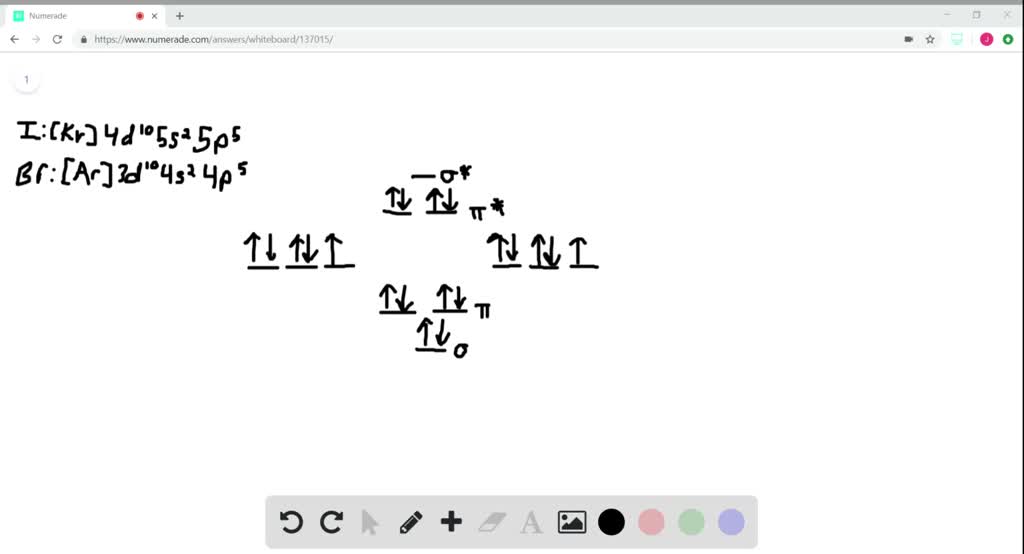
All these come with the chemical formula R2. 1)write the symbol for the metal and its charge followed by the symbol for the nonmetal and its charge. Other elements commonly found as diatomic molecules are fluorine (F 2), chlorine (Cl 2), bromine (Br 2), and iodine (I 2).
#Diatomic iodine charge generator
Trending Questions Will bromine undergo sublimation? Why does aluminum most often have an oxidation state of 3? Is a 93.3 degree temperature OK for a human? Which sphere do tornadoes belong in? What is the source for mechanical motion supplied to a generator called? Coniferous forest growing season? Colour of alluvial soil? Why does opposite poles on a magnet attract each other? What is the only liquid in the halide family? What is specifically does the Richter scale measure? Is carbon invisible? If A flask contains 440 mol of liquid bromine Determine the number of bromine molecules in the flask? What is hardness of st52. The diatomic elements are nitrogen, oxygen, fluorine, chlorine, bromine, iodine, and hydrogen. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas H 2, O 2, and N 2, respectively.


 0 kommentar(er)
0 kommentar(er)
